Finally!
A Mealtime Insulin Patch
Designed to Keep Up with Your Life.
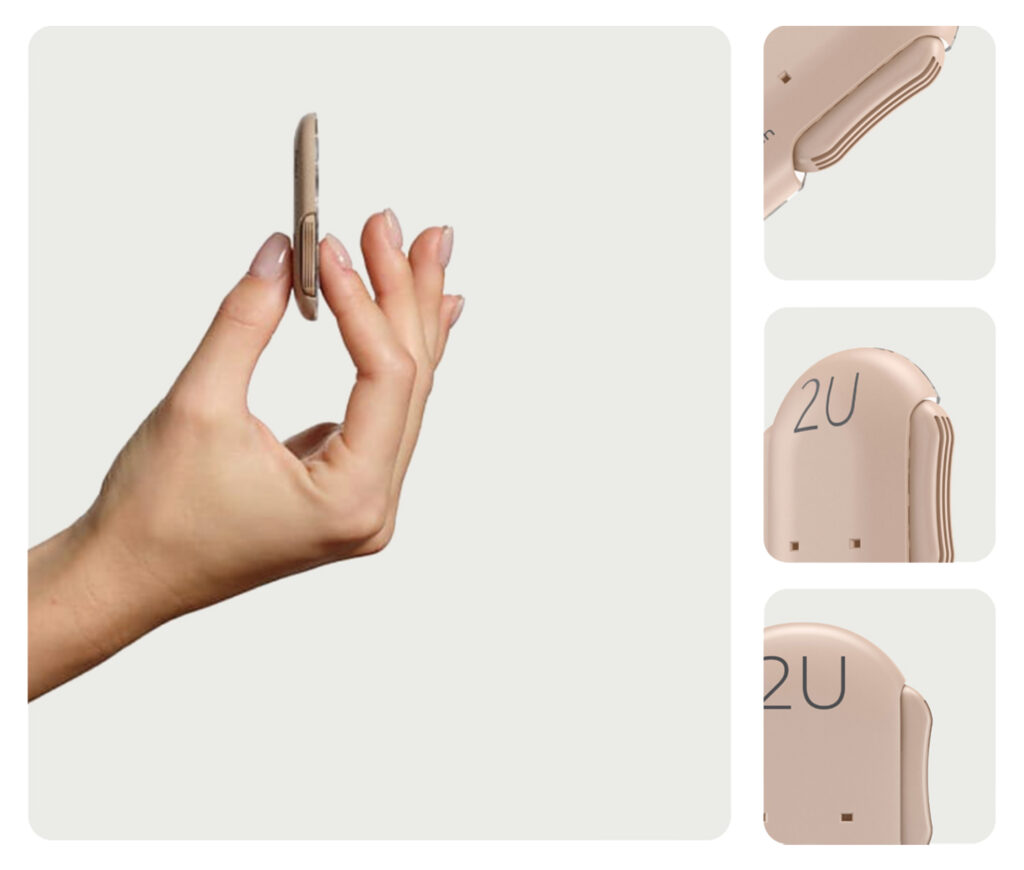
Fewer painful injections
Less hassle at mealtime
Easier than a pen
Discreet dosing
Improved glucose control
Encourages adherence
Better time in range
Thin and lightweight
Simple and easy to use
Finally!
A Mealtime Insulin Patch
Designed to Keep Up with Your Life.
Finally!
A Mealtime Insulin Patch
Designed to Keep Up with Your Life.
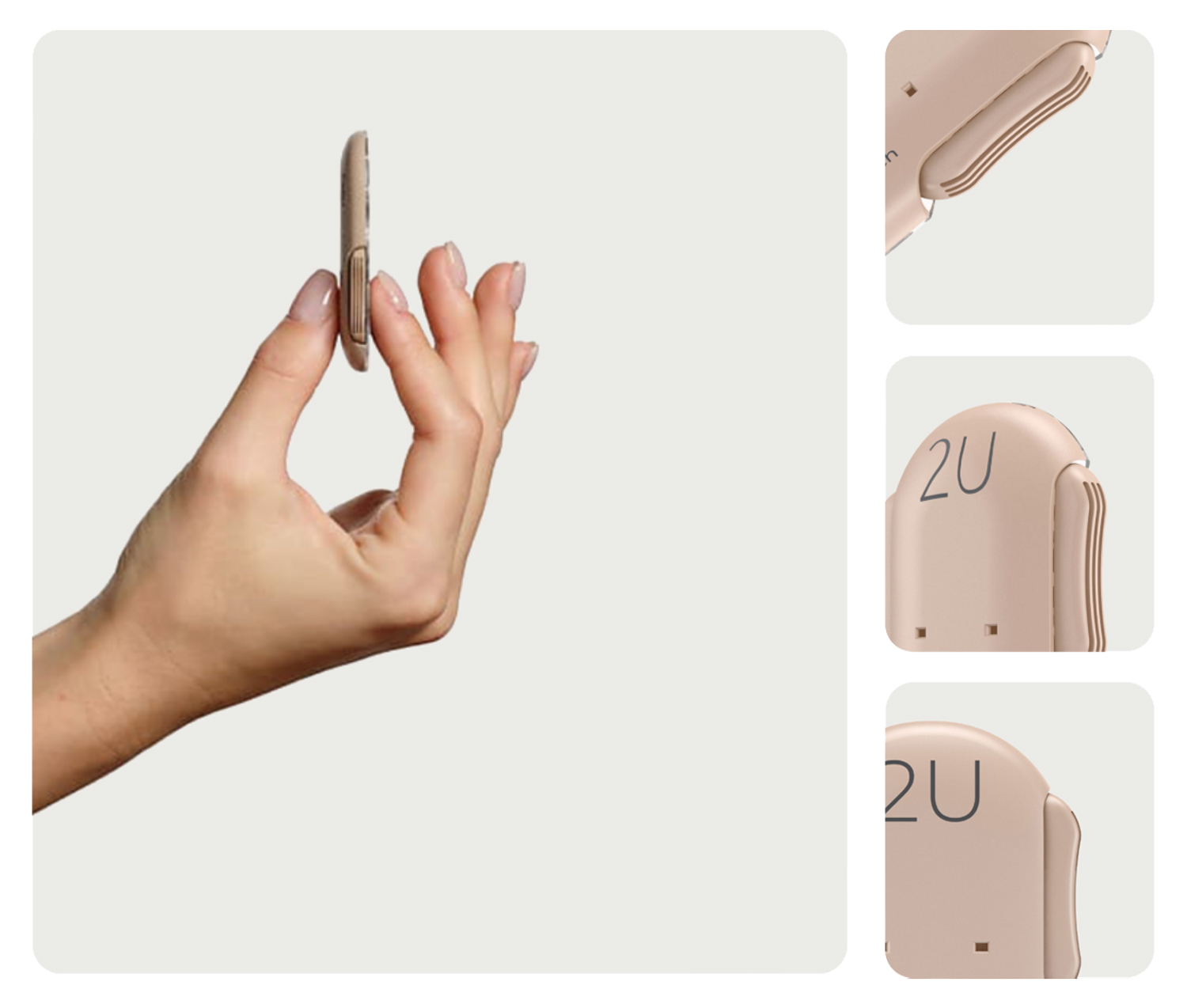
Fewer painful injections
Less hassle at mealtime
Easier than a pen
Discreet dosing
Improved glucose control
Encourages adherence
Better time in range
Thin and lightweight
Simple and easy to use
On-Demand, Wearable Insulin with CeQur Simplicity on Your Side.
On-Demand, Wearable Insulin with
CeQur Simplicity on Your Side
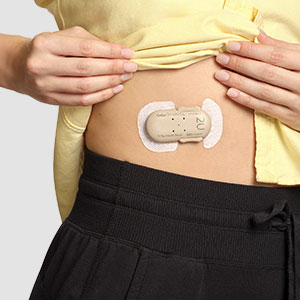
Convenient
One click delivers 2 units of rapid-acting insulin

Discreet
Compact, lightweight, and easily hidden under your clothes
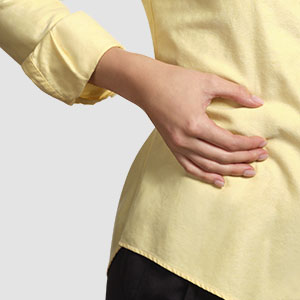
Injection-Free
Soft cannula, less pain, no hassle1
CeQur Simplicity is a patch that delivers injection-free mealtime insulin. It’s not a pen. It’s not a pump. It’s a wearable insulin patch! Using a tiny, flexible cannula, you can deliver rapid-acting insulin under your skin. It changes the way you dose for meals and correction boluses, eliminating the need for multiple daily injections.2

Easy Insulin
With CeQur Simplicity, you can click and dose in any situation. There is no complicated programming, no reliance on digital technology, and no exposed tubing. It’s simple, on-demand, one-click dosing for mealtime insulin and bolus correction.
CeQur Simplicity Is Clinically Shown to Keep Your A1C and Time-in-Range (TIR) in Check, Which Reduces the Risk of Long-Term Health Complications.1,2
Take back glycemic control
Flatten blood sugar spikes
Achieve your targets
CeQur Simplicity is Clinically Shown to Keep Your A1C and Time-in-Range (TIR) in Check, Which Reduces the Risk of Long-Term Health Complications1,4
Take back glycemic control
Flatten blood sugar spikes
Achieve your targets
CeQur Simplicity users decreased their A1C by an average of 1.7% after 24 weeks1
Results were comparable to pens. Consistent A1C <7% supports HEDIS Comprehensive Diabetes Care measure
References



